
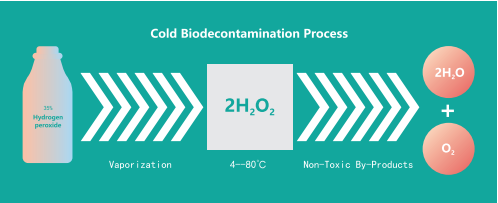
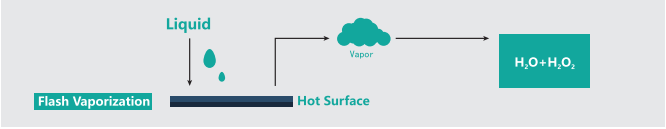
VHP is a technology that vaporizes the liquid hydrogen peroxide into hydrogen peroxide vapor and uses the vaporized hydrogen peroxide (VHP) to sterilize the surface of the object at low temperature.
VHP has broad-spectrum bactericidal properties and can effectively kill all types of microorganisms such as bacteria, fungi, molds, viruses, and bacterial spores. The most difficult microorganism to kill by VHP is found to be bacillus stearothermophilus, so the biological indicator used for VHP sterilization validation is bacillus stearothermophilus.
VHP sterilization is non-toxic and residue-free. The vaporized hydrogen peroxide can quickly kill microorganisms during the sterilization process, and then quickly degrade to H2O and O2 after sterilization, which is non-toxic and residue-free, while the residue concentration of hydrogen peroxide is detect-able.
VHP sterilization can be validated. A normal validation cycle includes parameter development, VHP distribution study, biological challenge test and exhaust degradation study. The TKSAGE-HPB VHP equipment has a complete GMP validation documentation system.
VHP sterilization has good compatibility. The TKSAGE-HPB series of VHP sterilizers use a unique saturation control method to ensure that the hydrogen peroxide does not liquefy or condense during the entire sterilization process, resulting in better material compatibility.
Standard procedure:
LOGA program: Using the process control method of D-value for sterilized microorganism and LOG reduction value for sterilization, the sterilization process is steadily adjusted to achieve the set sterilization conditions, which is generally set to 6LOG program.
LOGB program: Using the process control method of D-value for sterilized microorganism and LOG reduction value for sterilization, the sterilization process is steadily adjusted to achieve the set sterilization conditions, which is generally set to 12LOG overkill program.
Concentration program: The sterilization process is controlled by concentration and time, and the program is set according to the sterilization concentration and time conditions obtained from the parameter development to achieve the set sterilization conditions.
Self-cleaning procedure: Self-cleaning inside the cavity is achieved through the filtration of HEPA filter, to realize the transfer without sterilization, partially replacing the function of the self-cleaning pass box.
Introduction to common media:

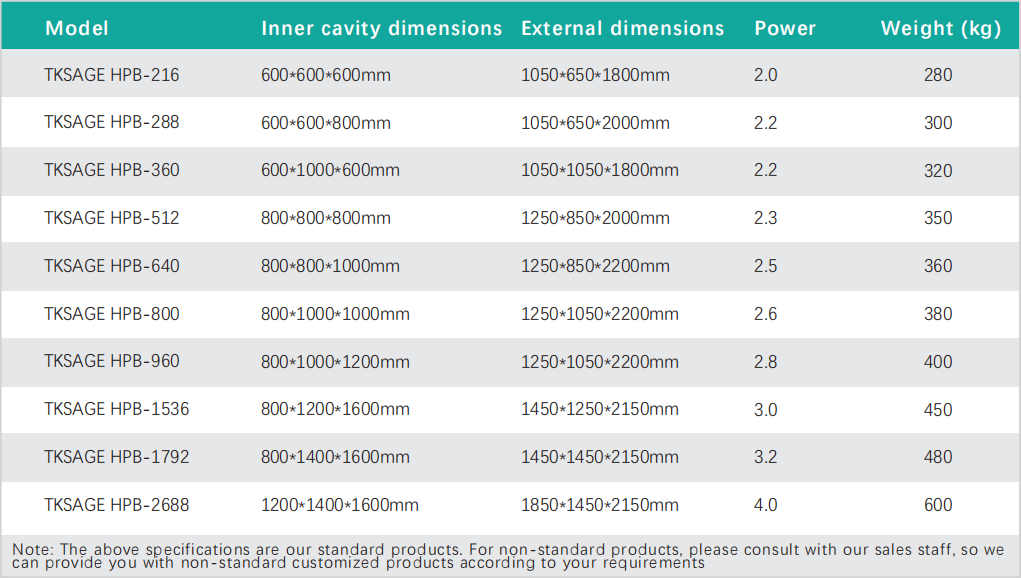
Specifications:
Sterilization process:
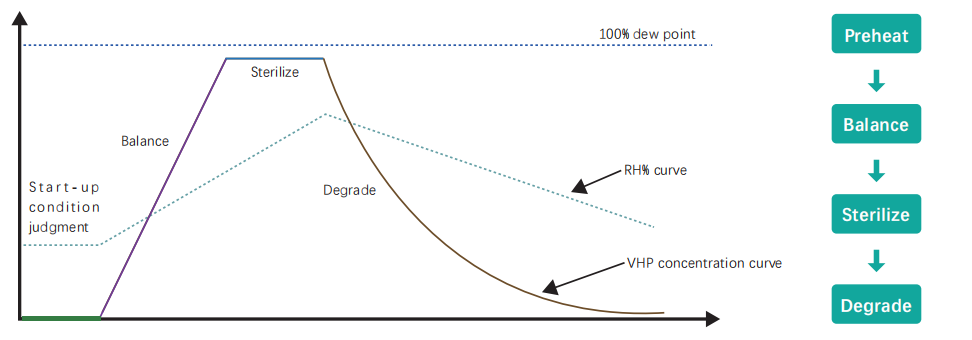
Preheat: The temperature and humidity conditions of the cavity are automatically adjusted before the equipment starts to reach the set program start conditions.
Balance: Activate sterilization conditions and the equipment performs self-balancing of VHP concentration and saturation to achieve sterilization conditions.
Sterilize: Start sterilization and accumulate the LOG value of sterilization until the end of sterilization.
Degrade: After the sterilization, the equipment enters the exhaust degradation stage for VHP residue removal and degradation until the end of the program.




